
Clean sanitary facilities efficiently and safely!
Keeping sanitary facilities clean is important for both visual and hygienic reasons. Because of their high moisture level and warmth, sanitary facilities are the ideal place for the growth of bacteria and fungi, which is why it is extremely important to clean sanitary facilities regularly and thoroughly.
When cleaning sanitary facilities, it is helpful to use acidic cleaning agents
Sanitary facilities create a lot of organic matter such as dead skin cells, hair, and fecal matter, but also mineral matter such as remnants of calc and minerals. When organic and mineral matter combine, surfaces will begin to rust. Unfortunately, this will not be removed just by a light cleaning, but rather, is needed to use aggressive acidic cleaning agents. Nevertheless, acidic cleaning agents should be handled with care, as wrong use will result in harming the cleaned surface and putting your health at risk.
What are acidic cleaning agents?
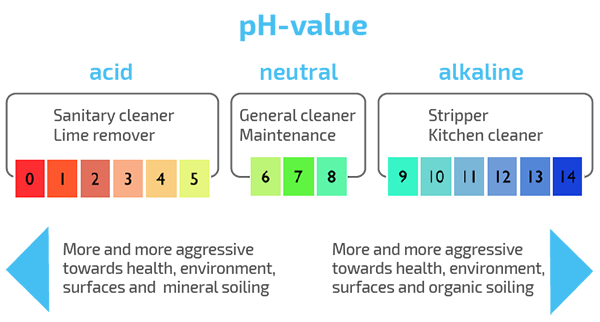
All cleaning agents are divided into three big groups according to their pH level: neutral, alkaline, and acidic. In addition to that, acidic substances are divided into three:
- Lightly acidic: pH 4,9 – 6
- Acidic: pH 2-4,9
- Strongly acidic: pH below 2
Lightly acidic cleaning agents are prophylactic, which means that they prevent the appearance of water sediments and remove dirt. Strongly acidic cleaning agents are of help when there is more serious sediment of rust, urine, and calc on the shower floor and walls, and also in the toilets and urinals. Acidic and strongly acidic cleaning agents should only be used as needed, as to some extent these agents are dangerous for both the surfaces and for human health. Excellent tool to remove reluctant dirt is, for example, Kiehl Sanpurid-Citro concentrate, and Kiehl Powerfix-Gel detergent.
At the same time, pH is not the only thing to look for when choosing an acid. Acids have different compositions and, if possible, it is suggested to always choose a cleaning agent produced based on natural acid. Even if a substance is strongly acidic based on its pH level, cleaning agents based on a natural acid are not as aggressive on the surfaces. Cleaning agents based on natural acid are for example:
Use acidic cleaning agents carefully
Since acidic cleaning agents can be dangerous to both surfaces and people, it is extremely important to follow the right safety technique while using them.
- Always wear rubber gloves when using an acidic substance
- Before using an acidic cleaning agent, be sure to wet the surfaces, especially the joints – this prevents the acid from harming the surface
- An acidic substance is always put in cold water, otherwise, substance particles will start flying and that is harmful to the health
- After working with acidic cleaning agents, remove the acid remnants from the surfaces by rinsing with a lot of water
When working with acidic substances, it is important to protect your hands with chemistry-proof nitrile gloves. For everyday cleaning with weaker acids, single-use nitrile gloves will do, but for special cleaning work with stronger acids, be sure to choose thicker and multiple-use nitrile gloves. You will find the exact requirements that the gloves must meet and how long they provide protection in the safety data sheet for the specific substance.
How to prevent calc
For surfaces with regular contact with water, such as faucets, plates, and sinks, the appearance of calc is unavoidable, but the easiest way to prevent it is by cleaning the surfaces after use. On larger surfaces such as walls and floor, use floor or wall cleaners, and for faucets and sinks, use a microfiber towel. That way, there is a less frequent need for acidic agents.










